Signed in as:
filler@godaddy.com
Signed in as:
filler@godaddy.com

The Fromme Lab is a prominent research group focused on elucidating the intricate mechanisms that regulate the Golgi apparatus, a vital organelle in eukaryotic cells. Specifically, their research centers on the interplay of small GTPases and their regulatory proteins, Guanine Nucleotide Exchange Factors (GEFs) and GTPase Activating Proteins (GAPs), in controlling cisternal maturation. This work not only contributes to our fundamental understanding of cellular biology but also holds significant implications for human health and the advancement of basic science.
Ari's Principle Investigator (PI) Chris Fromme, was a post-doctoral scholar in the lab of Randy Schekman, one of the 2013 Nobel Prize winners in Physiology and Medicine for his work on the Golgi!
The Golgi apparatus is a complex and highly organized organelle situated in the eukaryotic cell's endomembrane system. Its fundamental function is to process, modify, sort, and transport proteins and lipids synthesized in the endoplasmic reticulum (ER) to their specific cellular destinations. This organelle is central to cellular homeostasis, as it ensures the proper functioning of vital cellular processes, including secretion, signal transduction, and membrane trafficking. Dysregulation of Golgi function has been linked to various human diseases, such as neurodegenerative disorders, cancer, and congenital disorders of glycosylation. Therefore, understanding Golgi regulation is paramount for both basic science and human health research.
One of the key processes under investigation in the Fromme Lab is cisternal maturation. The Golgi apparatus consists of a series of flattened membrane-bound sacs called cisternae. Cisternal maturation is a dynamic process in which the Golgi cisternae progress through a maturation process, transforming from cis to medial to trans cisternae as cargo molecules move through the Golgi. This sequential maturation allows the Golgi to sort and modify proteins and lipids efficiently. It is regulated by small GTPases, including Arf and Rab proteins, and their corresponding GEFs and GAPs.
The Fromme Lab investigates how GTPases, GEFs, and GAPs orchestrate cisternal maturation and Golgi function. They seek to elucidate the molecular and cellular mechanisms that govern the spatiotemporal activation and inactivation of these regulators, as well as their roles in cargo sorting and vesicle trafficking within the Golgi apparatus. By understanding the precise molecular details of these processes, they aim to uncover the fundamental principles governing Golgi regulation.
The Golgi apparatus is a central hub for cellular processes and the sorting of critical molecules. Dysregulation of Golgi function can lead to severe health consequences, making this research highly relevant to human health. By uncovering the intricacies of Golgi regulation, the Fromme Lab's work has the potential to shed light on the underlying causes of various diseases and inform the development of therapeutic strategies. Additionally, their research contributes to our basic understanding of cellular biology, offering insights into fundamental processes that drive cellular homeostasis and protein trafficking.
In summary, the Fromme Lab's research into the regulation of the Golgi apparatus, with a specific focus on cisternal maturation and the involvement of small GTPases and their regulators, has profound implications for human health, as well as the advancement of basic science in the field of cell biology. Their work not only enhances our comprehension of Golgi function but also has the potential to drive discoveries that may lead to innovative therapies for diseases associated with Golgi dysfunction.

Ari was a rotation student in Brooks Crickard's lab at Cornell University, where they studied DNA motor proteins in Homologous Recombination. Their project involved optimizing a cross-linking assay to analyze Rad54 homo-oligomers through cross-linking mass spectrometry (CLMS).

Ari conducted research in Marcus Smolka's lab, focusing on the phosphoproteome during the DNA Damage Response. They worked on determining cell-cycle defects in PPM1G knockdown cell lines.

In Carolyn Sevier's lab, Ari investigated the impact of redox stress on protein folding. Their project involved functional assays to understand how oxidation of cysteines modulates the Unfolded Protein Response (UPR) by examining the redox mediators Sec63 and Kar2 in S. cerevisiae.
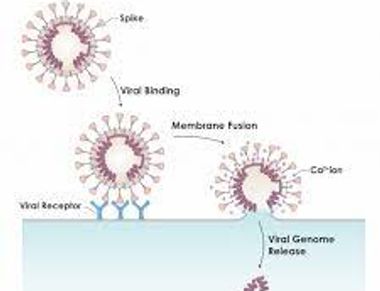
As a joint rotation student in Gary Whittaker and Susan Daniel's labs, Ari contributed to the study of coronaviruses. They conducted bulk membrane fusion assays to explore the influence of lipid composition on the membrane insertion of the SARS-CoV-2 fusion peptide.

In Elizabeth Kellogg's lab, Ari worked on optimizing advanced heterogeneous isolation protocols to enrich RNAPII in the pause state bound to chromatin, RNA, NELF, and other pause factors. This research focused on the structure of nucleic acid binding proteins.

As an undergraduate research assistant in Ewa Bienkiewicz's translational biophysics lab, Ari completed an honor's thesis project. They explored the binding affinities and cell membrane interactions of neuroprotective cellular prion protein (PrPC) octarepeat (OR) peptide mutants using techniques like surface plasmon resonance (SPR) and in vitro liposome assays.
.jpeg/:/cr=t:11.57%25,l:0%25,w:100%25,h:76.86%25/rs=w:388,h:291.72932330827064,cg:true)
During a summer research internship at Harvard University's Gaudet Lab, Ari investigated the substrate specificity of inducible gustatory receptor ion channels in insect orthologs and conducted mutagenesis experiments to explore substrate binding residues.

Ari worked as an undergraduate research assistant in Fanxiu Zhu's virology laboratory, where they studied Kaposi’s Sarcoma-Associated Herpesvirus (KSHV) and explored the role of KSHV ORF52 in viral infectivity and viral progeny production.

As part of the NSF Research Experiences for Undergraduates (REU) program, Ari was a research intern in Volker Vogt's lab. They compared the in vitro assembly of the structural protein Gag in Simian Immunodeficiency Virus (SIV) with the Gag protein in HIV using transmission electron microscopy and small molecule interaction assays, which led to their first publication.

During their participation in the NSF REU program at Louisiana State University, Ari was a research intern in Ping Wang's lab, where they characterized the morphologies of different clinical strains of the drug-resistant fungus R. delemar, contributing to the field of microbiology and immunology.
"The only true wisdom is in knowing you know nothing." -Socrates
Site Proudly Made by Ari Broad
Updated 2025 - All Rights Reserved
We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.